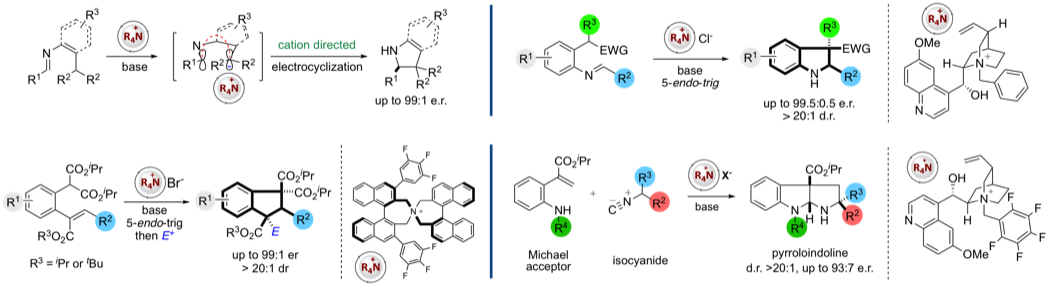
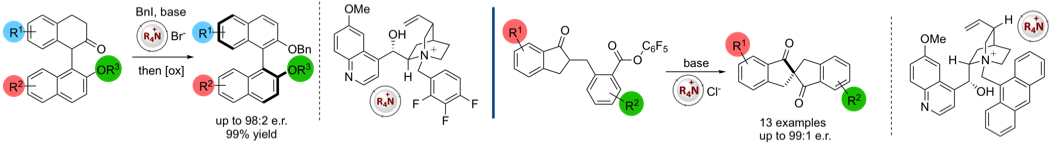
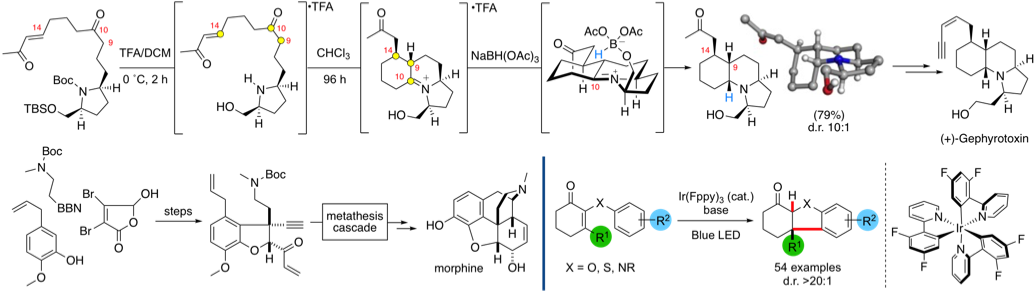
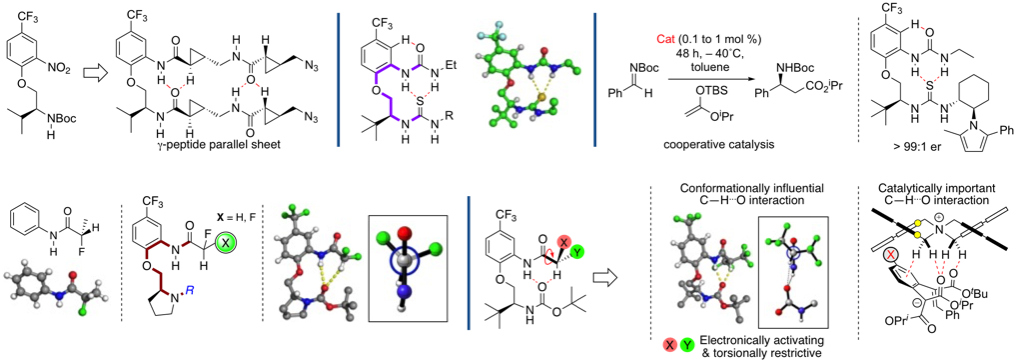
More recently our work has centred on approaches to control the synthesis of axially chiral molecules including axially chiral biaryls (via dynamic kinetic resolution or enantioselective SNAr reactions) and spirobiindanones (via direct enantioselective ketone C-acylation). We are also engaged in probing these reactions by kinetic profiling in conjunction with DFT calculations (in collaboration with Prof Robert Paton's group)